Dec 28, 14Chemical reaction Balancing chemical equations Home Reactions Blog Language ru es en Log in Chemical reactions Сhemical tables Potassium iodide react with hydrogen peroxide 2KI H 2 O 2 → I 2 2KOH Check the balance Potassium iodide react with hydrogen peroxide to produce iodine and potassium hydroxideHydrogen peroxide can be used to quickly oxidize soluble ferrous iron to ferric (Fe3), forming a rapidly settling ferric hydroxide floc The resulting floc can be removed with filtering or a clarifier This reaction is shown belowYes there are previous questions asking the same thing (one and two)However, the answers to both give only parts of the reaction, not the full equation

Question Video Using Word Equations To Describe The Decomposition Of Hydrogen Peroxide H2o2 Nagwa
What is the balanced equation for hydrogen peroxide
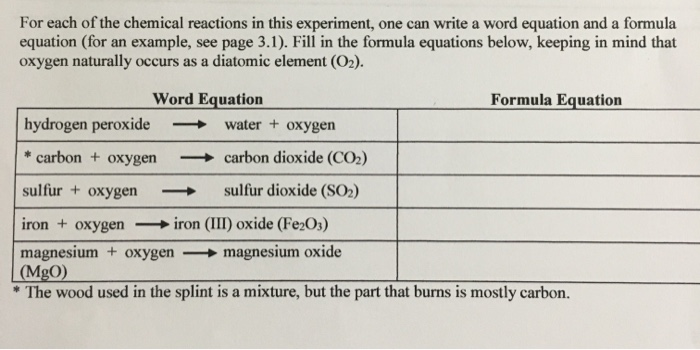
What is the balanced equation for hydrogen peroxide-How does catalase break down hydrogen peroxide?Water toegen Formula Equation 2H2 2H2O 02 10 What substances, other than oxygen, are in the generator when the decomposition of H,O, is complete?
.jpg)


Hydrogen Peroxide
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy &Dec , 152H_2O_2(l) rightleftharpoons 2H_2O(l) O_2(g) This is in fact a disproportionation reaction in that oxygen in peroxide (I oxidation state) has given water (II oxidation state for oxygen) and zerovalent oxygen gas (0 oxidation state for oxygen) Often a Mn^(2) salt is added to catalyze this reactionApr , Two molecules of hydrogen combine with two molecules of oxygen to form hydrogen peroxide
7 While there is no upper limit to the pH (eg, H2O2 can be used to dechlorinate effluent from caustic/chlorine odor scrubbers), as a practical matter, pH 85 is preferred in order to provide an instantaneous reactionQuestion Date 0926 Answer 1 In our body the enzyme catalase catalyses the reaction 2H 2 O 2 = 2H 2 O O 2, the decomposition of hydrogen peroxide into water and oxygenSo, how does catalase work?Oxygen and water 2H 2 O 2 =catalyst=>
Discussion The decomposition of hydrogen peroxide yields oxygen and water The reaction is catalyzed by the iodide ion ( I1) from KI (or NaI) as shown in the twostep process below The oxygen generated creates bubbles in the soap to produce a toothpaste like foam A glowing splint can be used to test that the gas produced is oxygenFirst recognized as a chemical compound in 1818, hydrogen peroxide is the simplest member of the class of peroxidesOf the several processes of manufacture, the principal ones involve reactions of oxygen from the air with certain organic compounds, especially anthraquinone or isopropyl alcoholMajor commercial grades are aqueous solutions containing 35, 50, 70, or 90 percent hydrogen peroxideH2O2 MnO2 (Hydrogen peroxide Manganese dioxide) H2O2 MnO2 (Hydrogen peroxide Manganese dioxide) Watch later Share Copy link Info Shopping Tap to unmute If playback doesn't



Hydrogen Peroxide Chemical Equation Formula
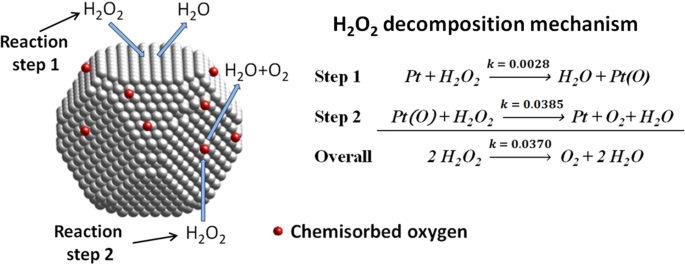


Kinetic Effect Of Surface Chemisorbed Oxygen On Platinum Catalyzed Hydrogen Peroxide Decomposition Springerlink
O 2 2H 2 O The hydrogen peroxide was used to provide a source of oxygen for the fuel in the torpedoes in many Russian submarines Hydrocarbon fuels don't burn underwaterAug 04, 15The balanced equation of the decomposition reaction of hydrogen peroxide is that 2H2O2 decomposes into the products 2H2O O2 (g) The resulting products are water and oxygen gas However, the decomposition takes place very slowly This is an experiment most students in chemistry lab are familiar with However, to make the reaction perceptible at a faster rate, aThe following equation describes the reaction between the permanganate ion and hydrogen peroxide in an acidic solution 2 MnO 4 (aq) 5 H 2 O 2 (aq) 6 H (aq) 2 Mn 2 (aq) 5 O 2 (g) 8 H 2 O (l) It might be interesting to see what happens when this reaction occurs in a basic solution Practice Problem 6



Hydrogen Peroxide Formula Uses Britannica


Hydrogen Peroxide Chemical Equation Tessshebaylo
Describe how the decomposition of hydrogen peroxide produced oxygen gas in both of the videos Tell students that both of the demonstrations use a 30% hydrogen peroxide solution Typically the hydrogen peroxide you can by at the store is only 3% hydrogen peroxide Explain to students that the chemical formula for hydrogen peroxide is H 2 O 2This page uses frames, but your browser doesn't support themSep 10, 18Benzoyl peroxide is an organic compound which has the chemical formula C 14 H 10 O 4 It can be prepared from the reaction between benzoyl chloride and hydrogen peroxide The compound consists of two benzoyl groups that are linked by a peroxide group Benzoyl peroxide is known to have antibacterial properties and is widely used in the treatment
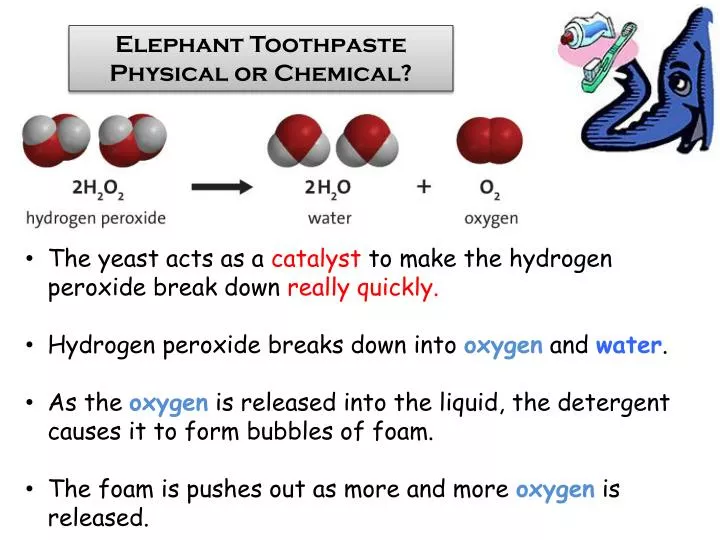


Ppt Elephant Toothpaste Physical Or Chemical Powerpoint Presentation Id
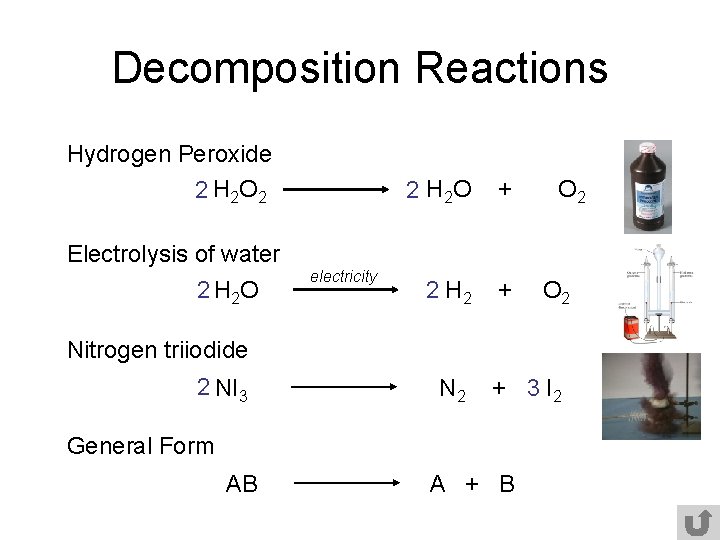


Chemical Equations Reactions Chemistry A Chemical Reactions You
Sep 08, 10Hydrogen peroxide naturally decomposes into H2O and O2 and does so faster in alkaline solution (your baking soda) 2H2O2 >WRITE IN THE CORRECT FORMULA THEN BALANCE THE CHEMICAL EQUATIONS ANSWERS Note Many elements exist as diatomic molecules ie H2, O2, N2, F2, Cl2, Br2, I2 1 Hydrogen Oxygen Water 2H2 O2 2H2O METALLIC OXIDE WATER BASE 2 Sodium oxide Water Sodium hydroxide Na2O H2O 2NaOH 3 Calcium oxide Water Calcium hydroxide CaO H2OThe decomposition of hydrogen peroxide in the presence of iodide ion occurs in two steps H 2 O 2 (aq) I (aq) = H 2 O (l) OI (aq) H 2 O 2 (aq) OI (aq) = H 2 O (l) O 2 (g) I (aq)



The Ideal Gas
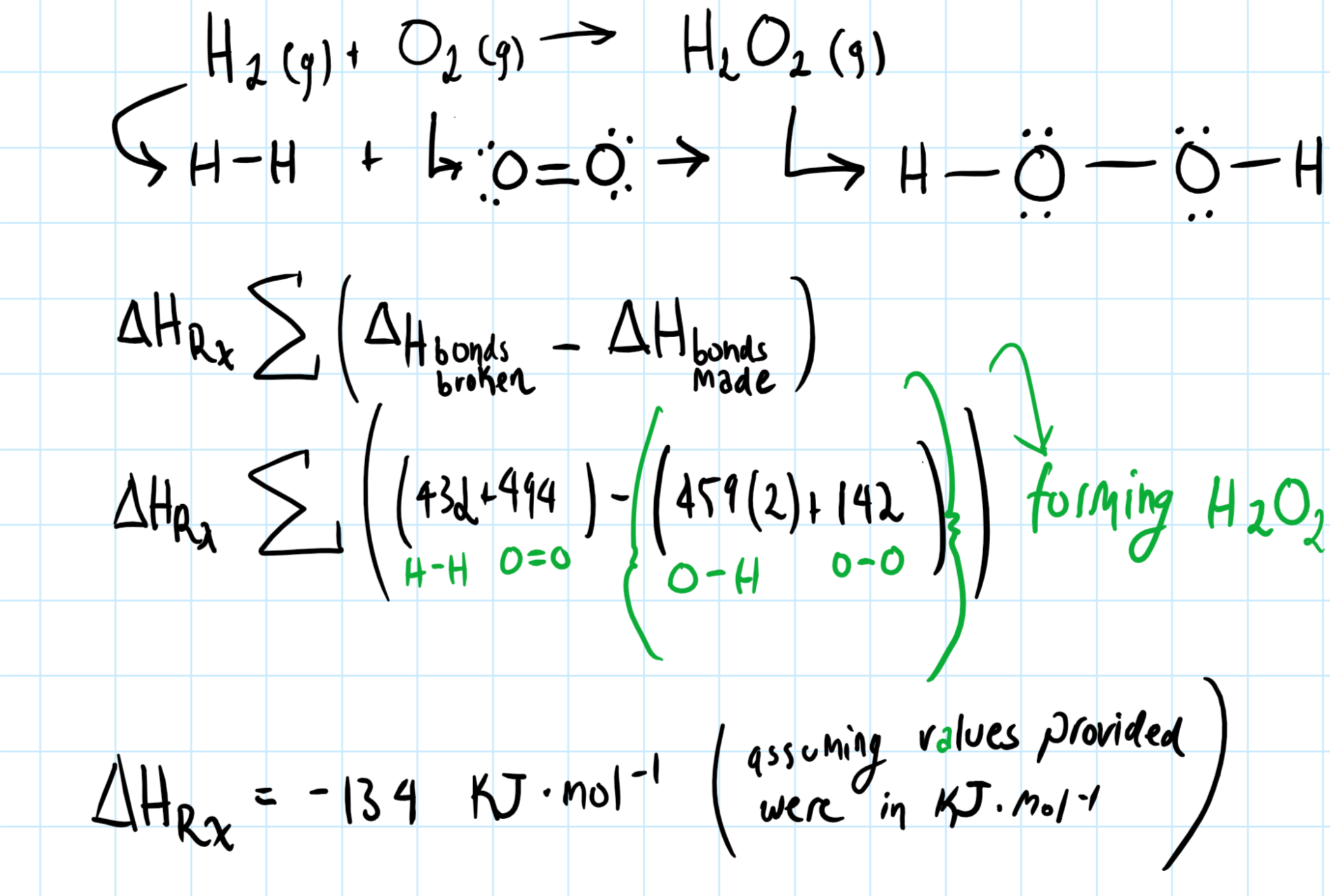


How Do You Determine The Heat Of Formation Of Hydrogen Peroxide From The Given Bond Energies H 2 G O 2 G H 2o 2 G Bond Energies H H 432 O2 494 O H 459 O O 142 Socratic
The Hydrogen Peroxide Breakdown 330 Laying the Foundation in Biology 7 Enzymes work by lowering the energy of activation For example, hydrogen peroxide decomposes to form water, H2O, and oxygen gas, O2 While this is a catabolic reaction, the rate at which it occurs is slowFeb 07, Exercise caution and/or use gloves when using the hydrogen peroxide (\(\ce{H2O2}\)) and the hydrochloric acid (\(\ce{HCl}\)) as they can cause chemical burns and skin irritation If either of these chemicals comes into contact with your skin, immediately rinse with water for a minimum of fifteen minutes and notify your instructor2 H2O}$$ But what exactly happens in melanin and which groups are oxidised is hard to say What probably happens is a series



Hydrogen Peroxide Formula Uses Britannica
.jpg)


Hydrogen Peroxide
2NaClO H2O2 >Catalase is a catalyst It exists in plant and animal cells and breaks down hydrogen peroxide, H2O2, which is a byproduct of metabolism Hydrogen peroxide is toxic if it accumulates in a cell The chemical reaction accelerated by catalase is written 2(H 2O 2) catalase 2H 2O O 2 Under favorable conditions, the reaction occurs very fastSafety How works Test new features Press Copyright Contact us Creators



Solved Report For Experiment 3 Continued Name 9 Write Chegg Com



Question Video Using Word Equations To Describe The Decomposition Of Hydrogen Peroxide H2o2 Nagwa
The formular for hydogen peroxide is H2O2 H 2 O 2 and it decomposes to form O2 O 2 and H2O H 2 O , ie oxygen and See full answer belowEquation (1) indicates that in an acidic solution, iodide ions are oxidized by hydrogen peroxide to triiodide ions These triiodide ions are reduced back to iodide ions by thiosulfate ions, equation (2) Reaction (2) is much faster than reaction (1) – it consumes triiodide ions as fast as they are formedHydrogen peroxide reacts with free available chlorine in solutions with pH >



Solution Hydrogen Peroxide H2o2 Decompo Clutch Prep


Hydrogen Peroxide Chemical Equation Tessshebaylo
NaCl H2O O2 This occurs in theory and is (I believe) fairly exothermic, producing bleach vapours as the oxygen gas bubbles out of solution I understand that the following may also occur depending on the concentration of peroHydrogen peroxide H2O2 CID 784 structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and moreNov 27, 19Hydrogen peroxide is a chemical compound containing molecules of hydrogen and water Its chemical formula is written as H 2 O 2 When hydrogen peroxide is in its pure form it is usually seen as a clear liquid with a slight pale blue colouration It has a higher viscosity than water
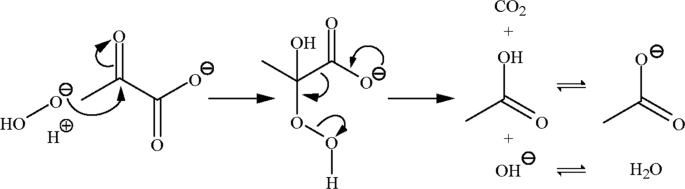


Reaction Rate Of Pyruvate And Hydrogen Peroxide Assessing Antioxidant Capacity Of Pyruvate Under Biological Conditions Scientific Reports


Hydrogen Peroxide Chemical Equation Tessshebaylo
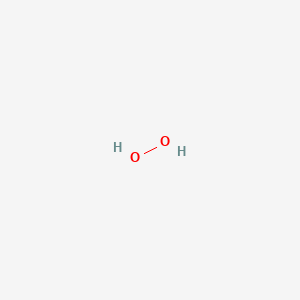
Hydrogen peroxide has the chemical formula H 2 O 2 and the following structural formula HOOH The hydrogen peroxide molecule contains one extra oxygen atom, compared to the more stable water molecule The bond between the two oxygen atoms, the socalled peroxide bond, is broken while two HO radicals are formedMar 25, Hydrogen peroxide molecule has molecular formula H2O2 having an interesting skew structural unit It is used widely in industrial solutions and antiseptic or mouth cleaner in medicine Due to the high oxidizing properties, it is used as a bleaching agentJul 18, 18The answer to this is number 2 2H2 = 2H2OO2 It is important that you know how to balance chemical equations to help you understand further why matter cannot be created and destroyed 2H2 is actually the scientific formula for Hydrogen Peroxide



Chemistry



Entry Quiz 1 1 What Is Chemical Reaction 2 Give An Example 3 What Is At The Left 4 What Is At The Right 5 What The Arrow Means Ppt Download
Potassium iodide and hydrogen peroxide reaction in acidic medium KI H 2 O 2 = I 2 H 2 O Potassium iodide is oxidized to iodine and hydrogen peroxide is reduced to water Also in the presence of excess potassium iodide produced iodine combines with iodide ion give red brown I 3 2KI H 2 O 2 H 2 SO 4 = I 2 2H 2 O K 2 SO 42H2O O2 I'd guess that the gas in your container was O2 gas that had been produced I know when you store stronger solutions of hydrogen peroxide, you need to keep it in a vented container because of O2 gas that builds upSep 19, 12Word equation Hydrogen Peroxide Water Oxygen_____ Formula equation 2H2O2 2H2O O2_____ 8 What substances, other than oxygen, are in the generator when the decomposition ofH2O2 is complete?MnO2 is the catalyst that is used so Mno2 will be left and also H2o(water)_______C Properties of Oxygen2


Hydrogen Peroxide Chemistry Ingridscience Ca


Redox Reactions
What is the full chemical equation when you use the following reactants to etch copper vinegar / acetic acid (5%) $\ce{CH3COOH (aq)}$ hydrogen peroxide (3%) $\ce{H2O2 (aq)}$ salt $\ce{NaCl (s)}$;Hydrogen peroxide is a highly reactive chemical containing the elements hydrogen and oxygen (H2O2) Pure hydrogen peroxide is a colourless liquid, but it is sold on the market as solutions in water, containing up to 33 – 37% pure hydrogen peroxide and other additives to prevent product decomposition In industry, it is mainly used in the production of chemicals and in bleaching ofThe protein has a certain 3D structure when it is active, which contains a channel into which the hydrogen peroxide can
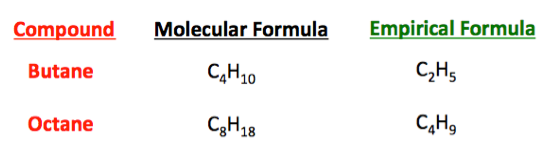


What Is The Empirical Formula Of H2o2 Socratic
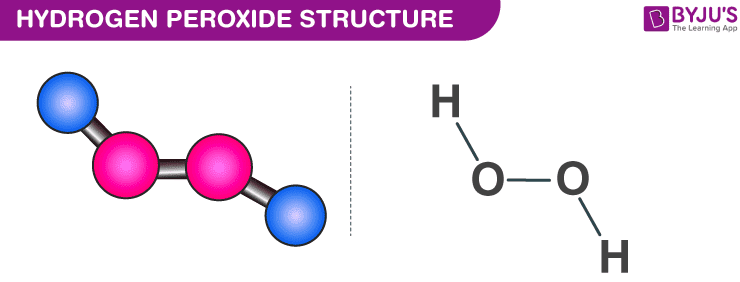


Hydrogen Peroxide H2o2 Structure Preparation Properties Uses
Hydrogen peroxide dosedependently increased the intracellular ROS generation, which was significantly repressed by HW, both in the cytoplasm and nuclei LIVE/ DEAD staining and our original cell viability dyeextraction assay showed that HW significantly protected HGF cells from hydrogen peroxide induced cell deathWrite the word and formula equations for the preparation of oxygen from hydrogen peroxide Word Equation Hydrogen peroxide >3 Balance polyatomic ions as a unit



Hydrogen Peroxide Formula H2o2 Over 100 Million Chemical Compounds Mol Instincts


Decomposition Of Hydrogen Peroxide In Alkaline Cyanide Solutions
Manganese peroxide C Properties of Oxygen 1Peroxide Gel Compositions US Abstract The present invention is the use of Poly(2ethyl2oxazoline) in the creation of peroxide gels for various applicationsSuch applications include bleaching of hair, teeth, laundry or any other bleachable item Blending of the gel is accomplished by mixing the Poly(2ethyl2oxazoline) with a peroxide such as hydrogen peroxide2 Add hydrogen peroxide and observe reaction Understanding In this reaction, the clear and colorless liquids, aqueous solutions of potassium iodide and hydrogen peroxide, are mixed to create a clear liquid with a brownish tint The product of this reaction is molecular iodine
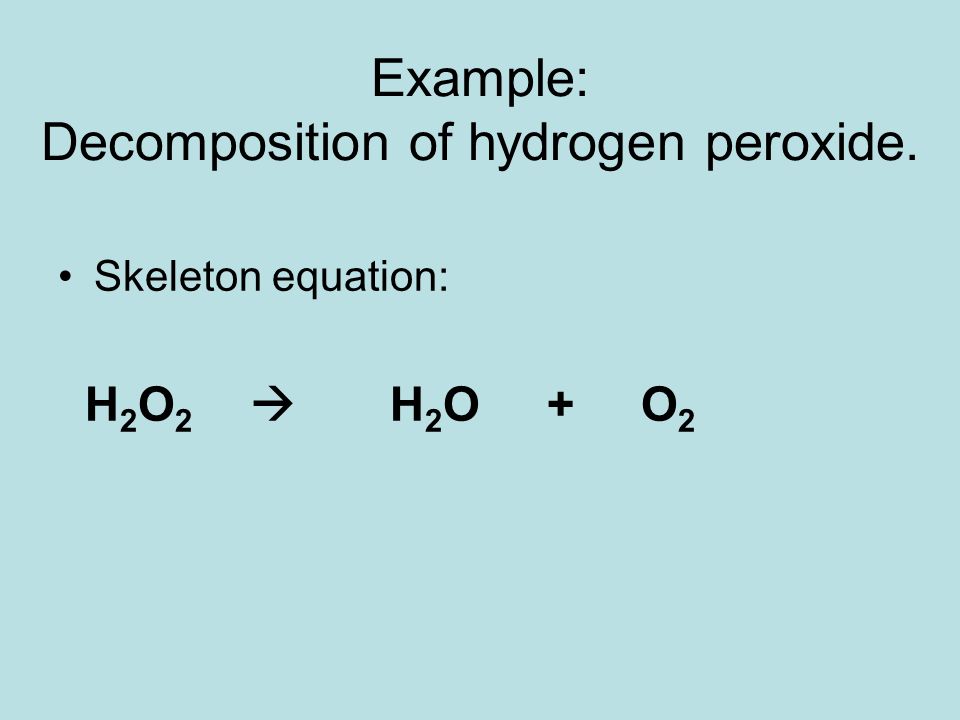


Ch 9 Chemical Reactions Equations Ppt Download



Doc Titration Of Hydrogen Peroxide Zaheer Abbas Academia Edu
Chemistry Hydrogen peroxide can act as either an oxidizing agent or a reducing agent, depending on the species present in solution Write balanced halfreaction equations for each of the following (a) H2O2(aq) acting as an oxidizing agent in an acidic solutionHydrogen peroxide Formula Hydrogen peroxide is a chemical substance very used as bleach, oxidizing and antimicrobial agent Formula and structure Hydrogen peroxide has the chemical formula H 2 O 2 Its molecular formula is H 2 O 2 and its molar mass is g mol 1Oxygen and water, hydrogen peroxide is said to be one of the most versatile and cleanest chemicals available Hydrogen peroxide was discovered in 1818 by Louis Jacques Thenard He reacted barium peroxide with nitric acid and then with hydrochloric acid He managed to obtain pure hydrogen peroxide, which was called oxygenated water


Hydrogen Peroxide Formula


Hydrogen Peroxide H2o2 Pubchem
An aqueous solution of lead(II) acetate is the byproduct of a 11 ratio of hydrogen peroxide and white vinegar (acetic acid) used in the cleaning and maintenance of stainless steel firearm suppressors (silencers) and compensators The solution is agitated by the bubbling action of the hydrogen peroxide, and the main reaction is the dissolutionTherefore, unfortunately there cannot be an exact description of what happened between melanin and hydrogen peroxide You can draw a halfequation for the hydrogen peroxide part which would be $$\ce{H2O2 2 e 2 H >Balanced Chemical Equation 2H2O2 → 2H2O O2



Hydrogen Peroxide Chemical Equation Tessshebaylo
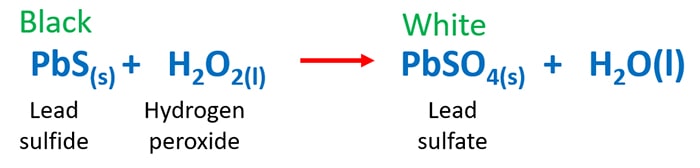


Pbs H2o2 Pbso4 H2o Lead Sulfide Hydrogen Peroxide Reaction
Feb 10, 21b Because one molecule of nheptane contains 16 hydrogen atoms, we need 8 H 2 O molecules, each of which contains 2 hydrogen atoms, on the right side \\ce{C7H16 (l) O2 (g) → 7 CO2 (g) } \underline{8} \ce{H2O (g) } \nonumber \ 16 hydrogen atoms on both reactant and product sides;



Hydrogen Peroxide Wikipedia



Hydrogen Peroxide Wikipedia
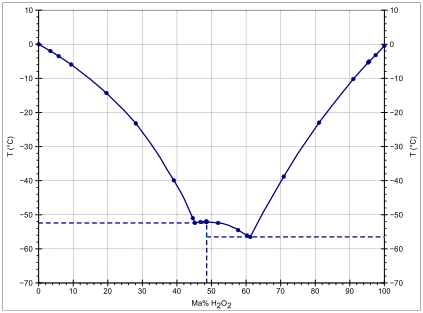


Hydrogen Peroxide Wikipedia
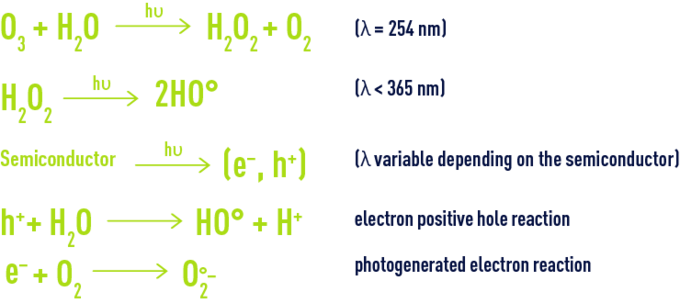


Water Treatment The Oxidants And Disinfectants Degremont



A Catalyst And The Rate Of Reaction Chapter 6 Chemical Change Middle School Chemistry


Chemical Oxygen Generation Respiratory Care


Residential Use Of Hydrogen Peroxide For Treating Iron And Hydrogen Sulfide Wcp Online



Pdf Decomposition Of Hydrogen Peroxide Kinetics And Review Of Chosen Catalysts



Quantitative Chemistry Part 2 Ppt Video Online Download
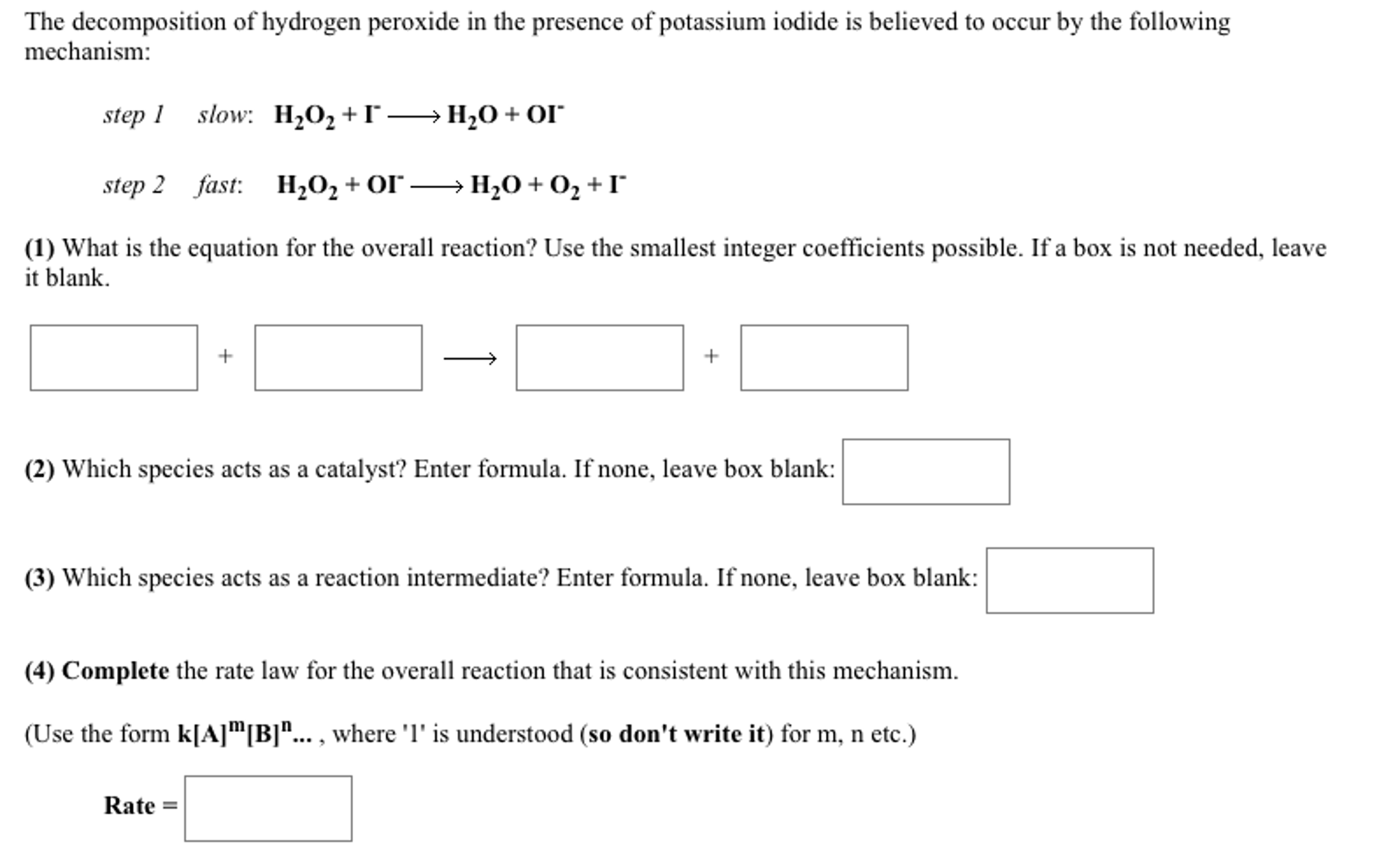


Solved The Decomposition Of Hydrogen Peroxide In The Pres Chegg Com


Hydrogen Peroxide H2o2 Chemspider
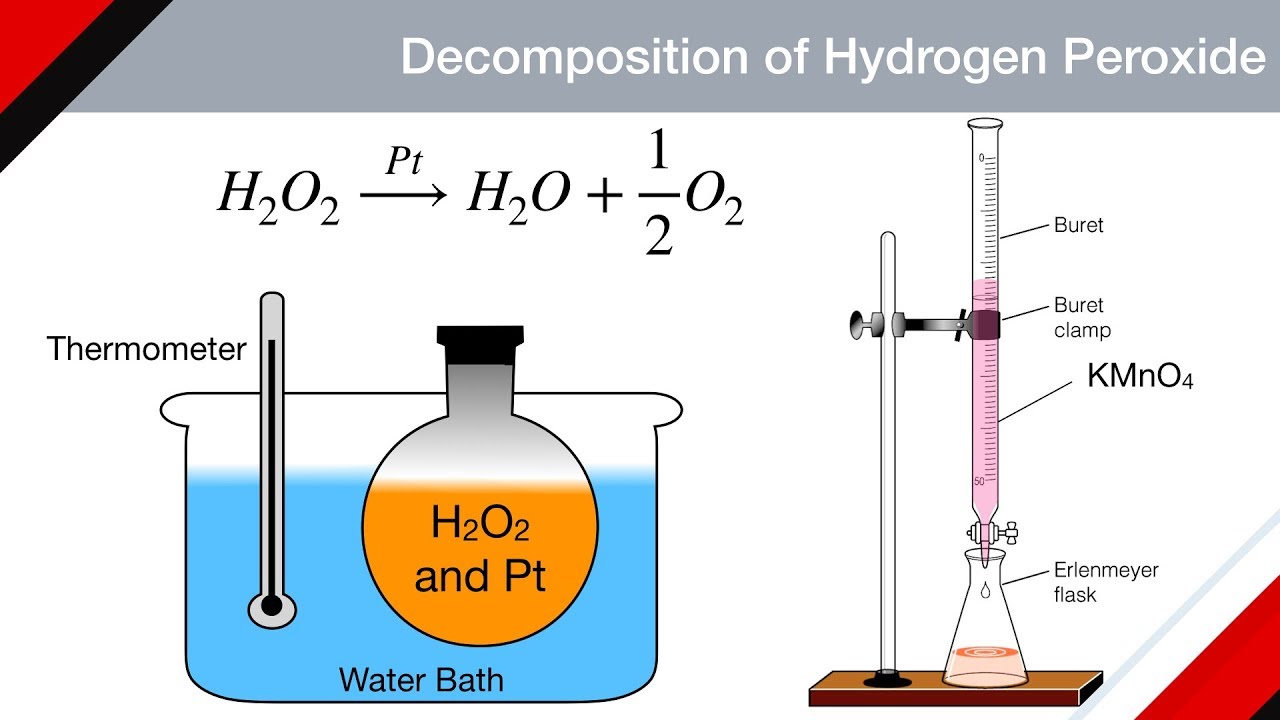


Kinetics Of Decomposition Of Hydrogen Peroxide Chemical Kinetics Physical Chem Youtube



How Do I Determine Which Is The Reduction Reaction In A Hydrogen Peroxide And Potassium Permanganate Solution Chemistry Stack Exchange



Hydrogen Peroxide Formula Uses Britannica



Bleach And Hydrogen Peroxide Reaction Balanced Equation Youtube



Hydrogen Peroxide An Overview Sciencedirect Topics


What Is The Name Of H2o2 Quora


Hydrogen Peroxide Chemical Equation Decomposition Tessshebaylo


H2o2 Mno2 Hydrogen Peroxide Manganese Dioxide Youtube
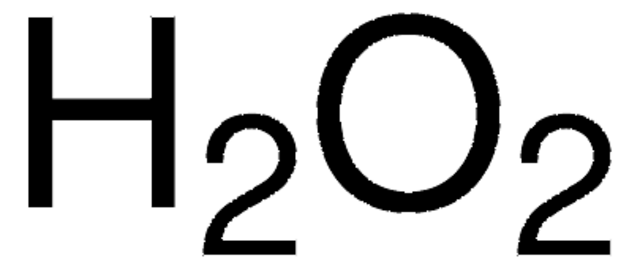


Hydrogen Peroxide 50wt Water Stabilized 7722 84 1 Sigma Aldrich



Solved An Aqueous Solution Of Hydrogen Peroxide Decompose Chegg Com


16 5b Hydrogen Peroxide H 2o 2 Chemistry Libretexts



Cassie Venable Alexis Teplick Hair Dye Chemicals In Hair Dye Nh 3 Ammonia H 2 O 2 Hydrogen Peroxide Structure At Right Shows The Dinitrodiphenylamine Ppt Download


Solved For Each Of The Chemical Reactions In This Experim Chegg Com
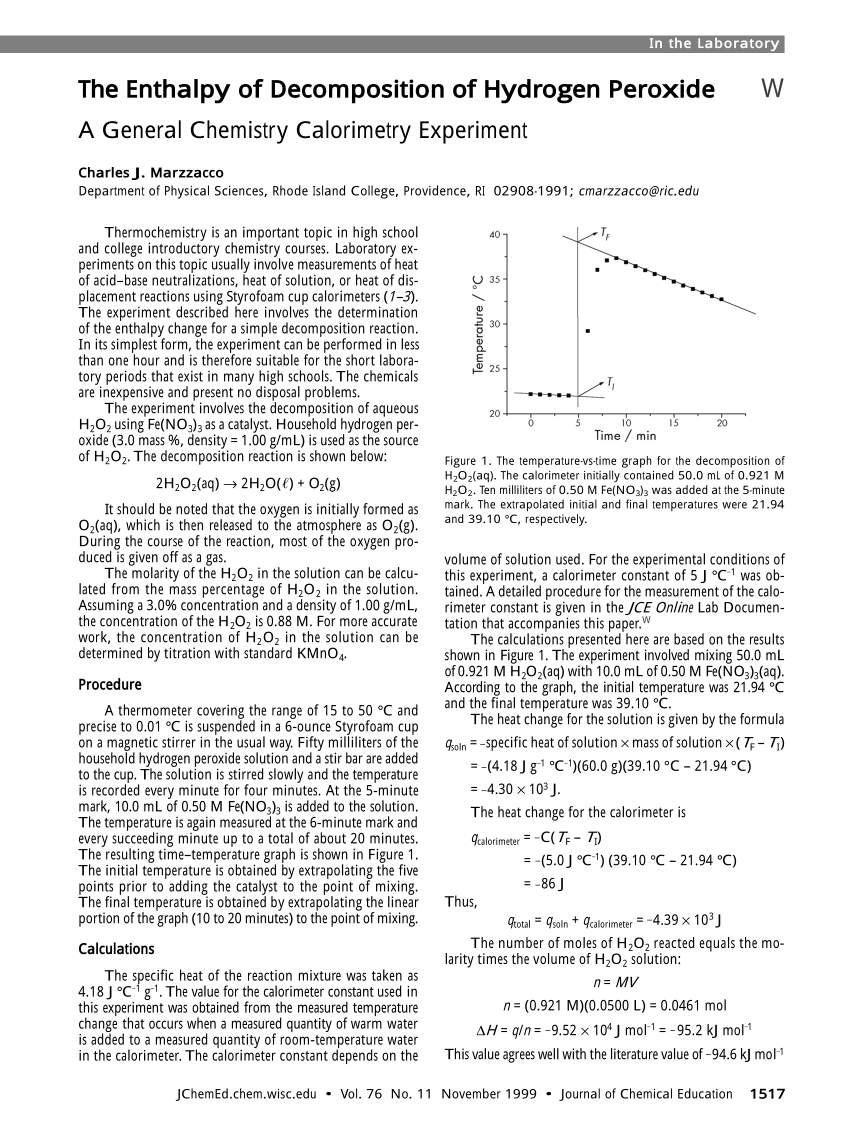


Pdf The Enthalpy Of Decomposition Of Hydrogen Peroxide A General Chemistry Calorimetry Experiment



H2o2 H2 O2 Balanced Equation Hydrogen Peroxide Water Oxygen Balanced Reaction Youtube


What Is The Reaction Between H2o2 And Mno2 Quora



Chemical Equation For Hydrogen Peroxide And Potassium Iodide Reaction Brainly In
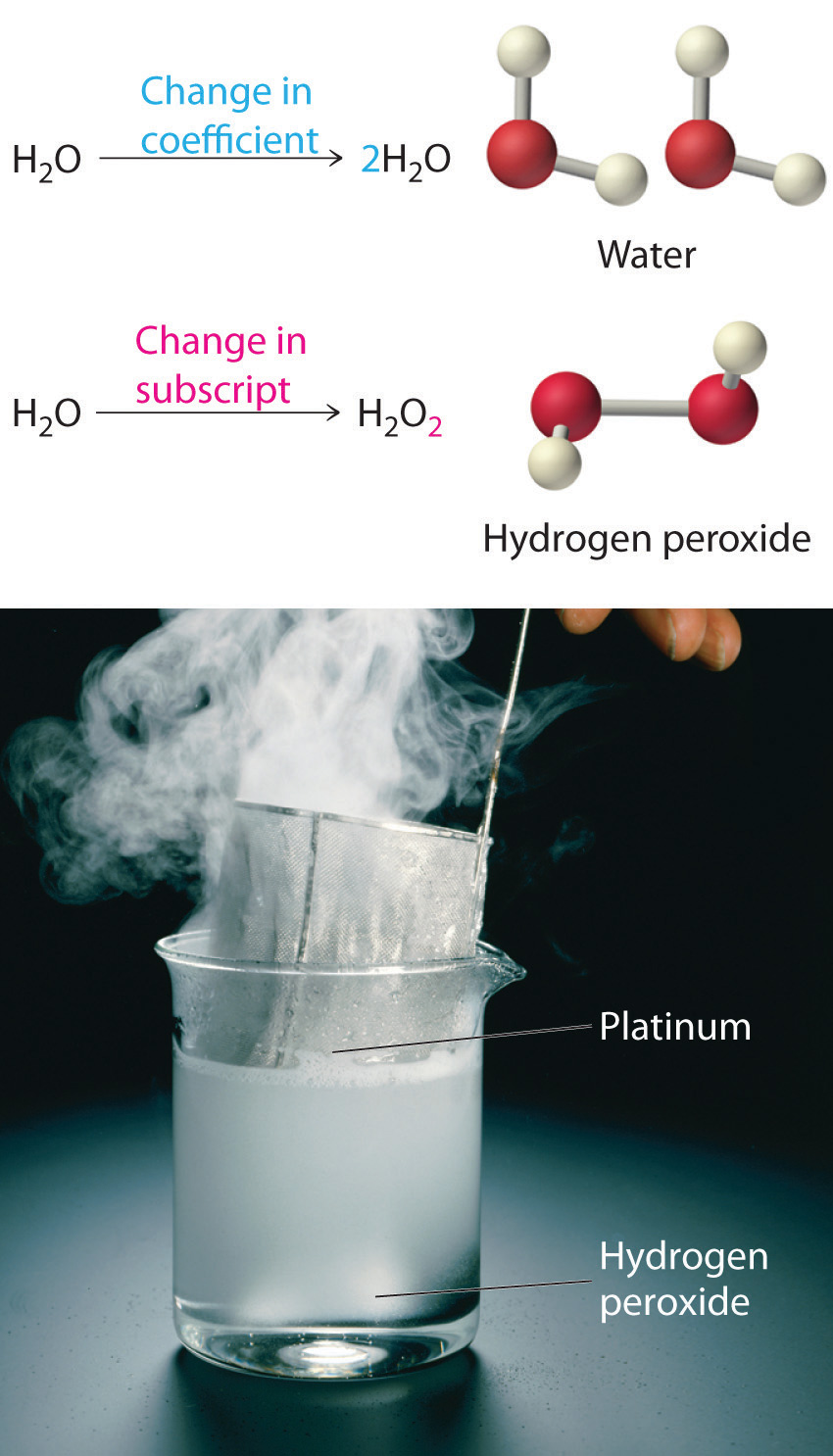


Chemical Equations



Effect Of Hydrogen And Propylene On The Hydrogen Peroxide Decomposition Over Pt Pto And Au Catalysts Sciencedirect
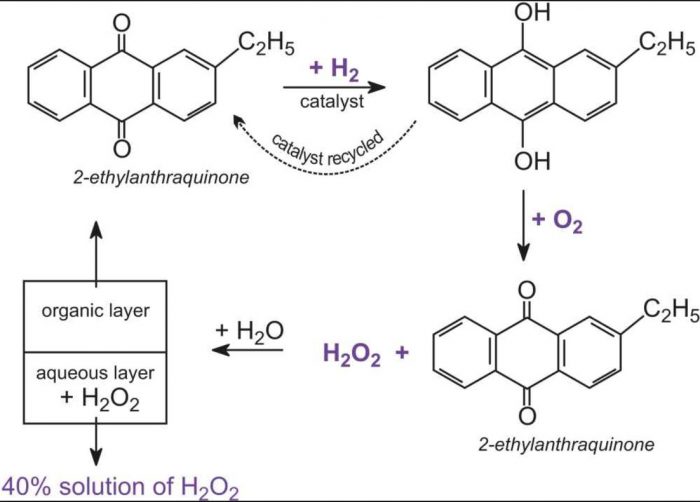


Hydrogen Peroxide Chemistry Class 11 Hydrogen



What Is The Empirical Formula Of H2o2 Socratic
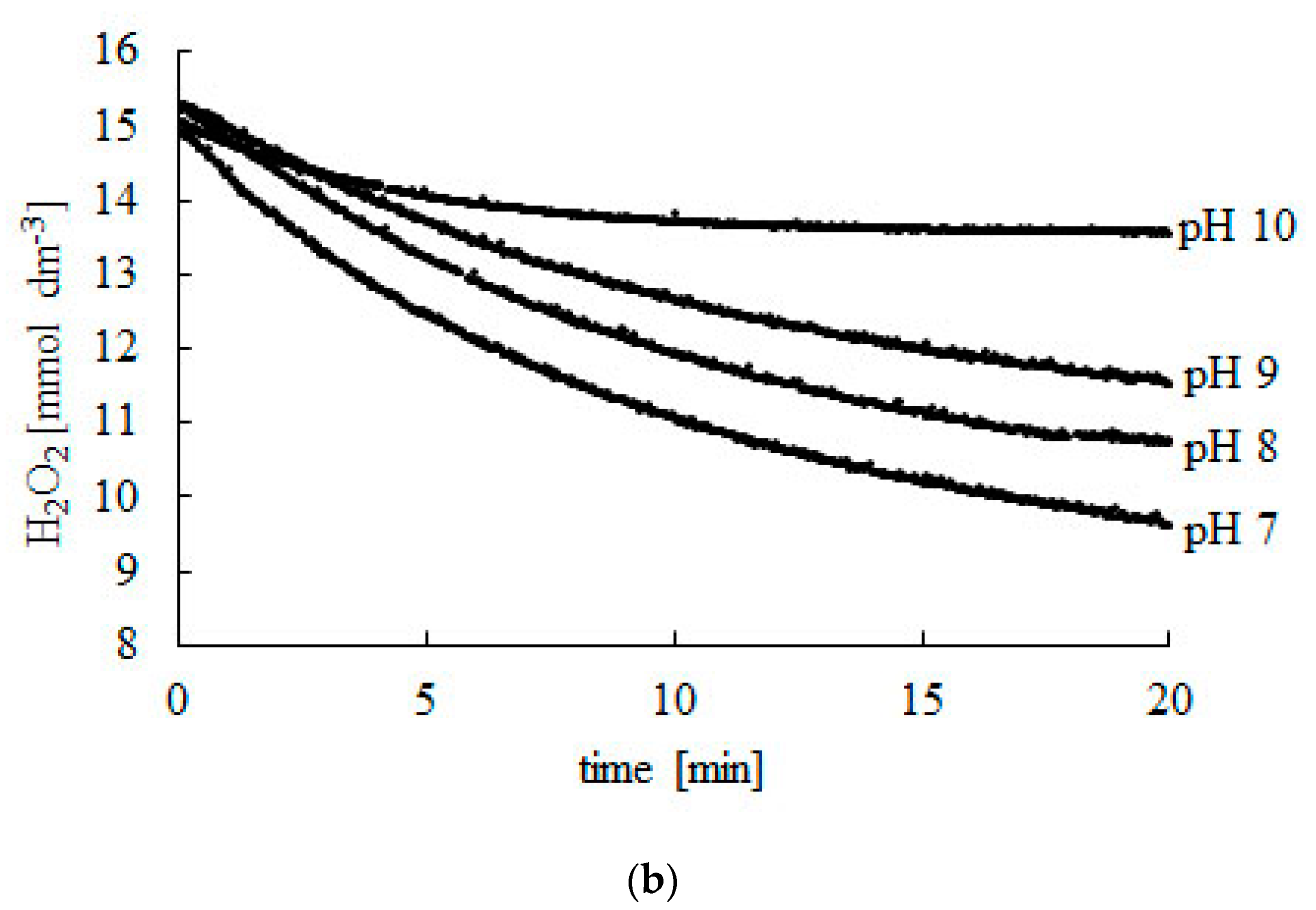


Catalysts Free Full Text New Method Of Determining Kinetic Parameters For Decomposition Of Hydrogen Peroxide By Catalase Html



Liquid Hydrogen Peroxide Decompose To Form Water And Oxygen Gas Write Balanced Chemical Reaction Brainly In



Hydrogen Peroxide Formula Uses Britannica


H2o2 Ki Hydrogen Peroxide Potassium Iodide Youtube


Carbamide Peroxide Ch6n2o3 Pubchem



Half Equation Hydrogen Peroxide Youtube


How To Balance H2o2 O2 H2o Decomposition Of Hydrogen Peroxide Youtube
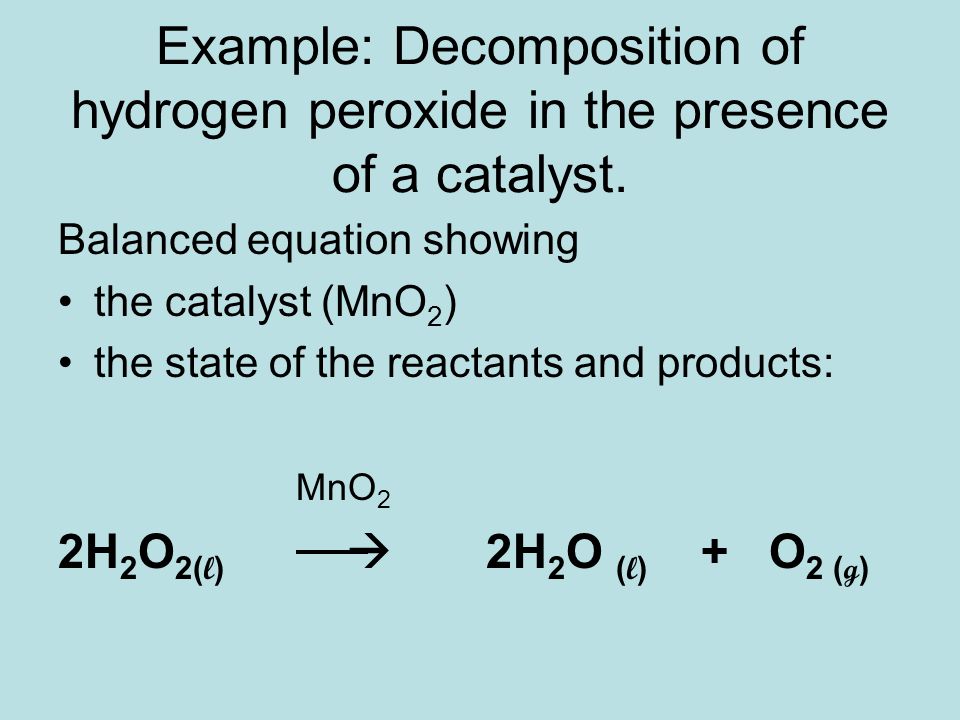


Ch 9 Chemical Reactions Equations Ppt Video Online Download
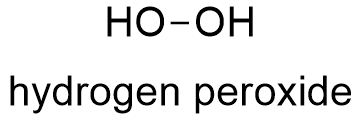


Hydrogen Peroxide Formula



Hydrogen Peroxide Hair Dye Glow Sticks Rocket Fuels Compound Interest



Hydrogen Peroxide And Bleach Balanced Equation Tessshebaylo



Hydrogen Peroxide Experiment By Lawrence Kok Issuu
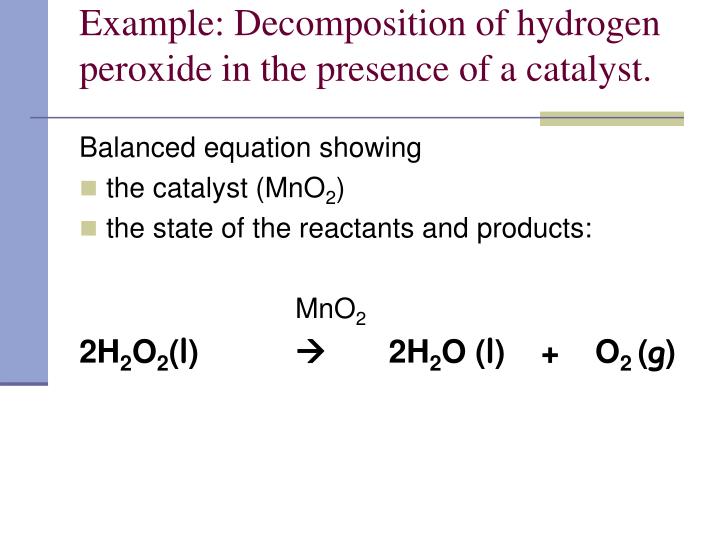


Hydrogen Peroxide Balanced Chemical Equation Tessshebaylo
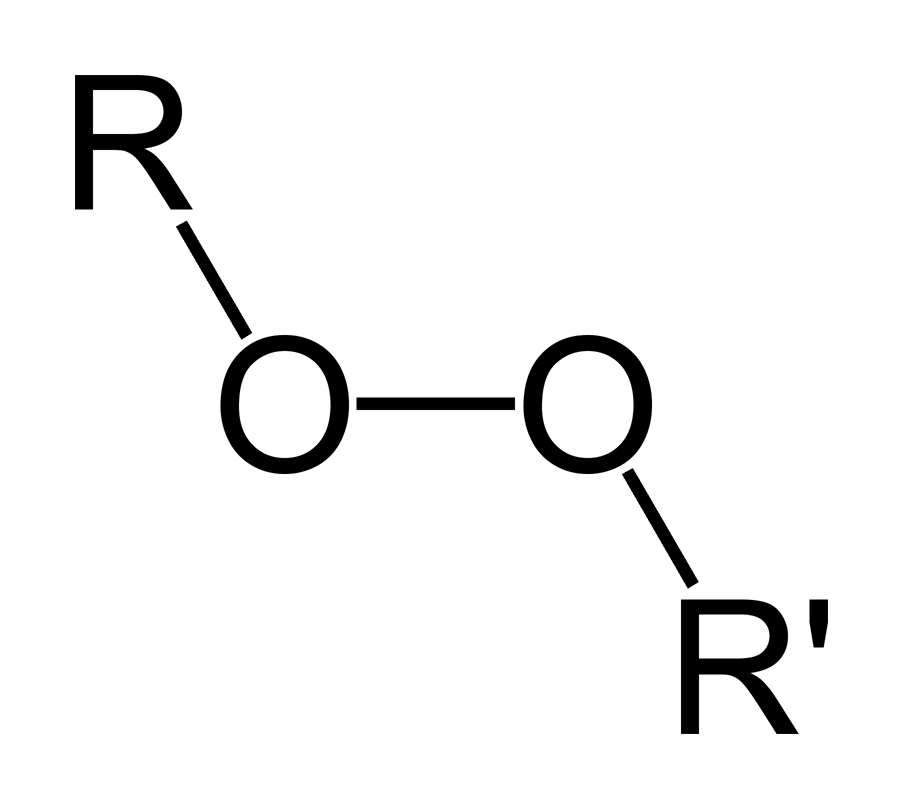


Organic Peroxide Wikipedia



Question What Is Hydrogen Peroxide Formula Bill Walters Online


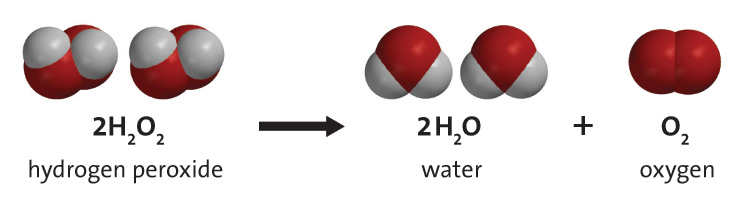
Hydrogen Peroxide Decomposes According To The Equation Below H 2o 2 L H 2o L 1 2 O 2 G Deltah Tutorke



How To Write The Formula For Hydrogen Peroxide Youtube



359 Questions With Answers In Hydrogen Peroxide Science Topic



Hydrogen Peroxide Formula Uses Britannica



Solved Report Sheet Decomposition Of Hydrogen Peroxide D Chegg Com



Question Video Using The Rate Of Decomposition Of Hydrogen Peroxide And The Balanced Chemical Equation For The Reaction To Calculate The Rate Of Formation Of H O And O Nagwa


How Does A Catalyst Make Hydrogen Peroxide S Decomposition Quicker What Is Actually Happening Socratic


Effect Of Temperature On Enzyme Action Brilliant Biology Student



Media Portfolio



Decomposition Of Hydrogen Peroxide Lab Answers Schoolworkhelper
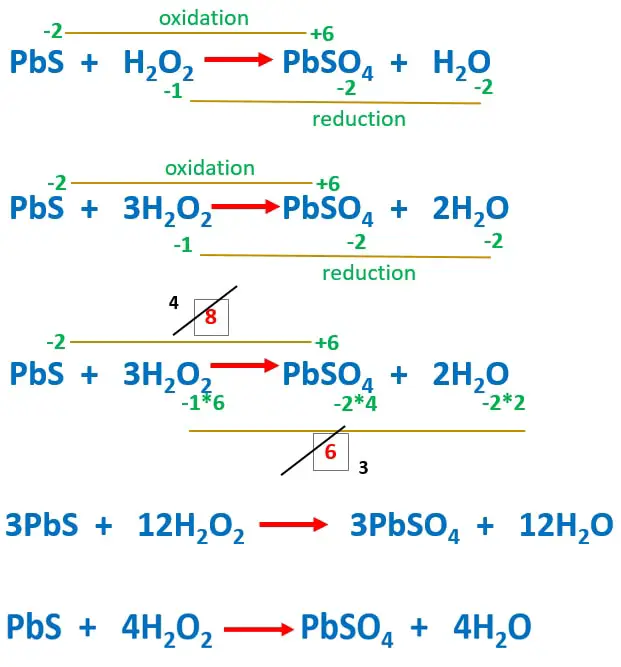


Pbs H2o2 Pbso4 H2o Lead Sulfide Hydrogen Peroxide Reaction



How To Make Oxygen From Permanganate And Hydrogen Peroxide Science Experiments Wonderhowto


An Acidic Solution Of Hydrogen Peroxide Behaves As An Oxidising As Well As Reducing Agent Illustrate It With The Help Of A Chemical Equation Sarthaks Econnect Largest Online Education Community



Hydrogen Peroxide Formula H2o2 Over 100 Million Chemical Compounds Mol Instincts



Hydrogen Peroxide Wikipedia
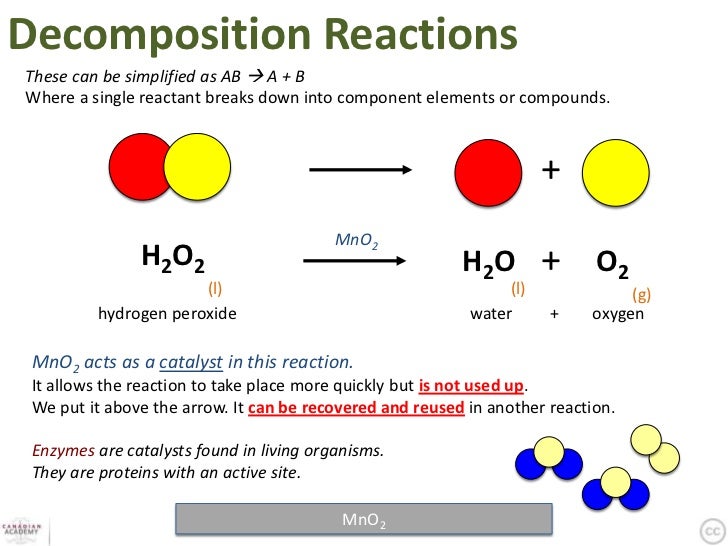


Reactions Formulas


Answered The Decomposition Of Hydrogen Peroxide Bartleby
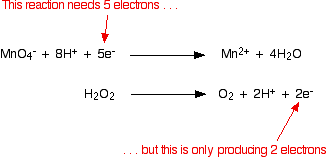


Writing Equations For Redox Reactions Chemistry Libretexts


Write A Balanced Chemical Equation For Hydrogen Peroxide Decomposes To Produce Water And Oxygen



Solved 1 Write Out The Balance Equation For The Decomposi Chegg Com



0 件のコメント:
コメントを投稿